A leading precision genetic medicine company focused on inner ear disorders
We are developing targeted adeno-associated viral (AAV) vector-based gene therapies for sensorineural hearing loss, the most common form of hearing loss and one of the most common of all sensory disorders.
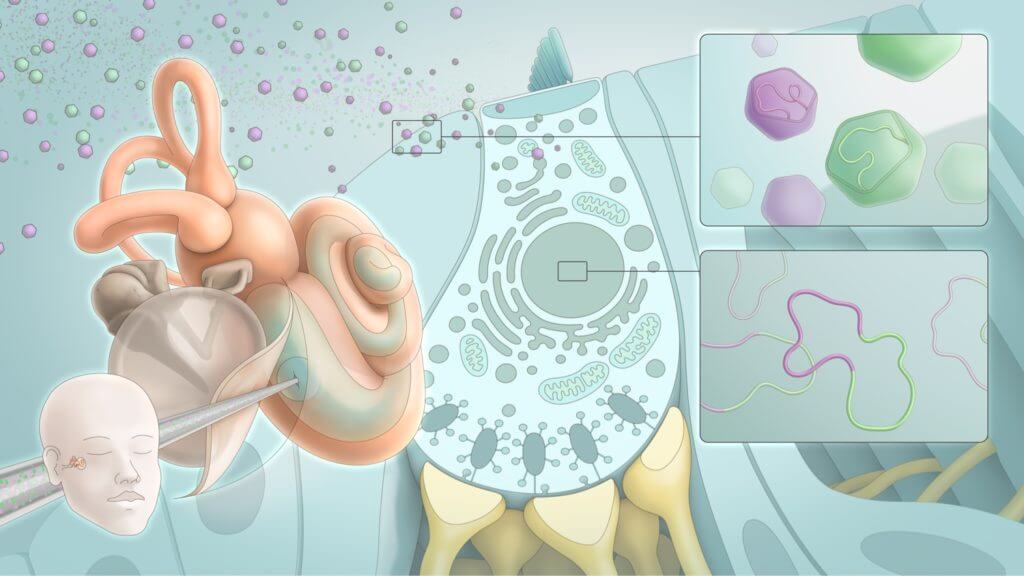
The Anatomy of Sound
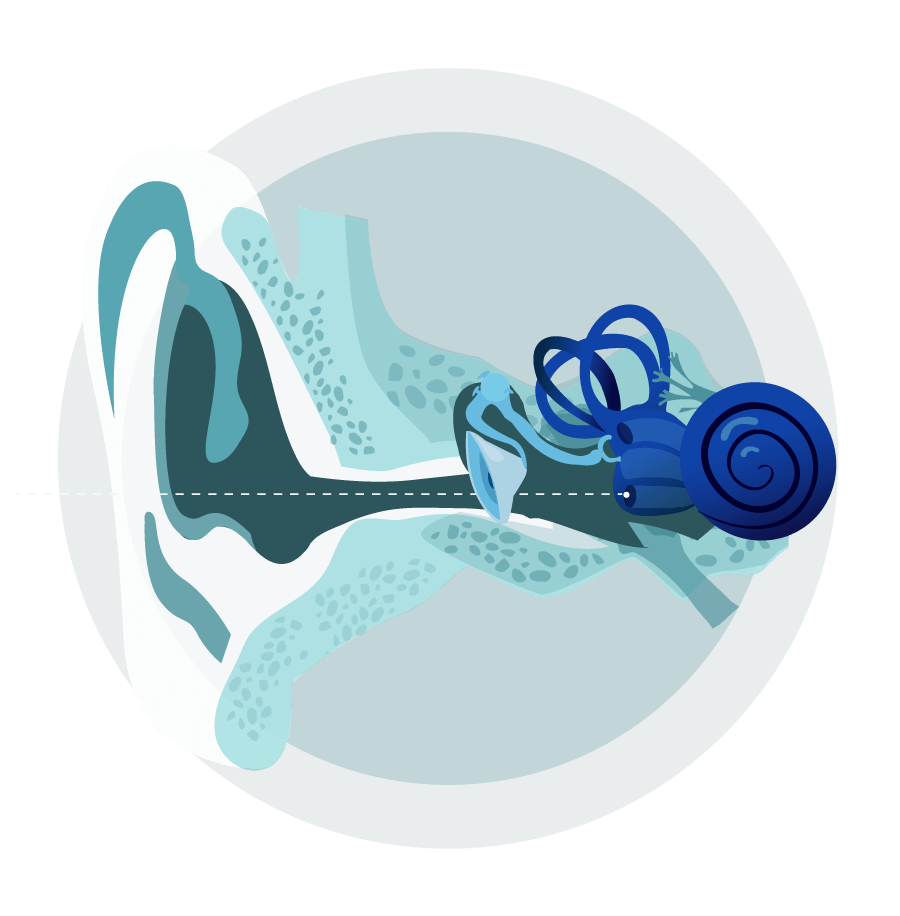
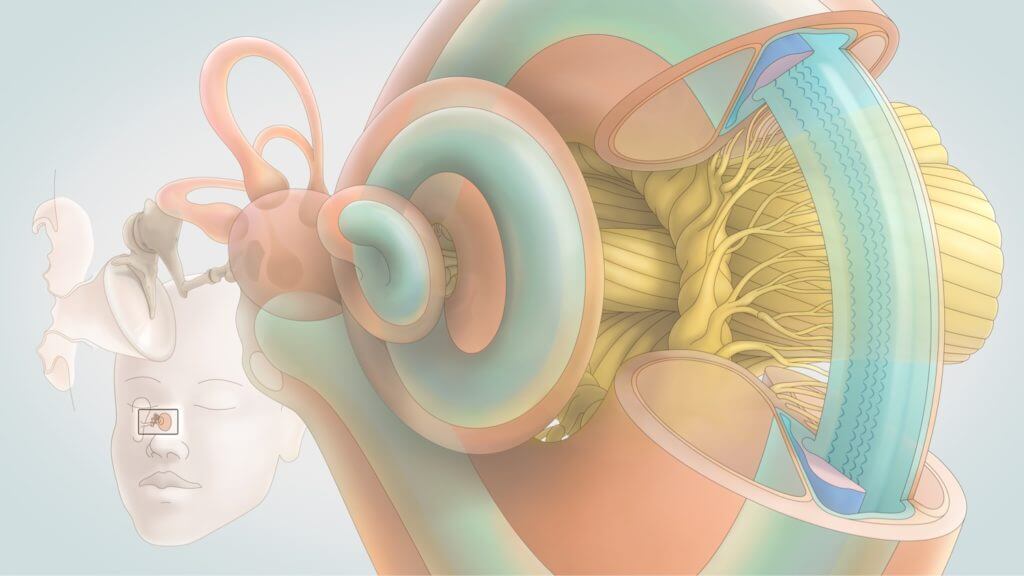
The ability to detect complex sounds is possible because of the fine structures of the ear, how they interact with each other, and how they connect to the brain.
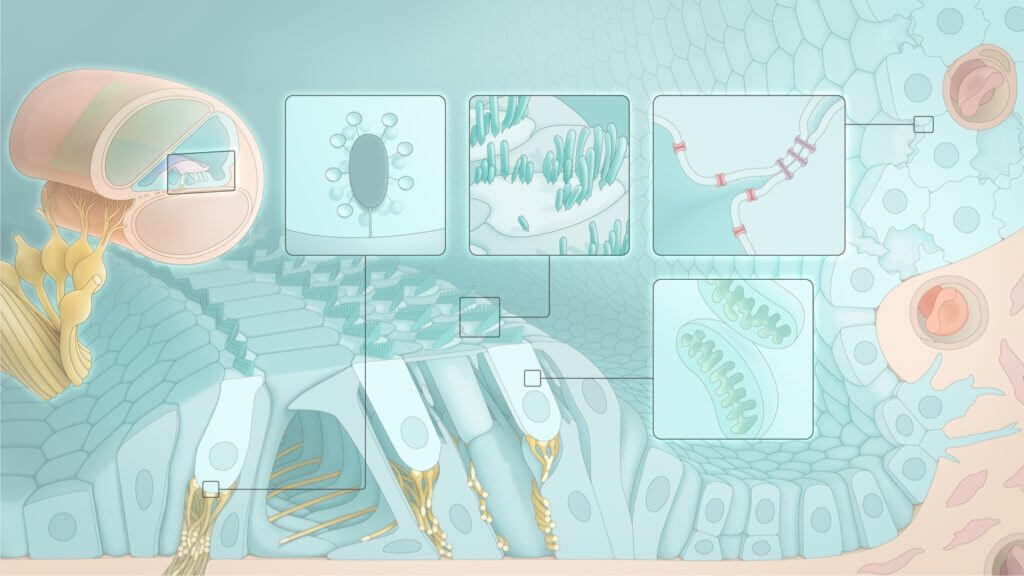
The outer ear helps capture air compression waves and funnels them to the eardrum. The eardrum is connected to the bones of the middle ear, which work together to convert air compression waves into fluid waves in the cochlea. The cochlea is the part of the inner ear that contains the sensory cells, which in turn convert fluid waves into neural impulses that can be transmitted to the brain. This final step in sound transduction requires the coordination of dozens of specialized cell types, each performing a specific function based on a unique gene expression profile. The resulting biology allows us to resolve inner ear movements 100,000 times smaller than the width of a strand of hair.
The intricate interplay of inner ear structures required for healthy hearing can be disrupted by changes in the DNA that encodes essential proteins and regulates their function.
Addressable Target Landscape
Millions of people worldwide are deaf or hard of hearing as a result of genetic mutations affecting any one of over 200 genes linked to inner ear conditions. There are more than 6,000 known variants across these genes that can result in cochlear pathology and subsequent hearing loss. Depending on the genes involved, various subpopulations of cells in the cochlea can be affected.
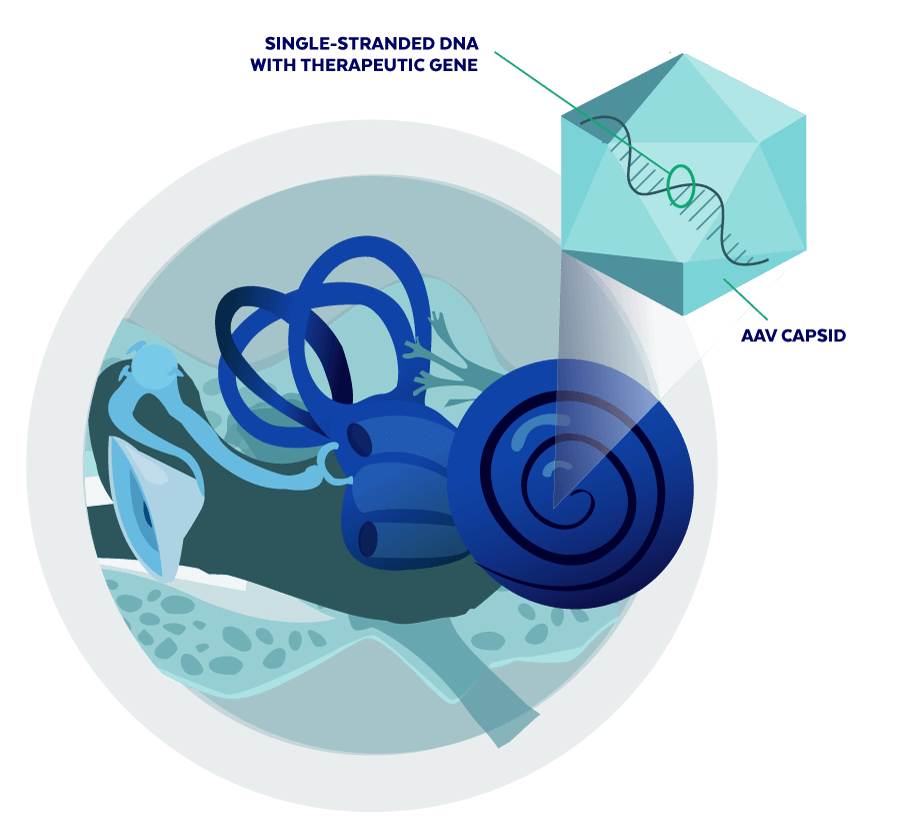
In some cases, it may be possible to recover hearing by delivering a healthy copy of the affected gene to the cells that need it.
What Akouos is Doing to Help
At Akouos, we are combining a novel delivery approach with the latest advances in gene therapy and hearing research with the goal of restoring the natural function of the inner ear. Akouos is advancing multiple candidate therapies that target sensory cells (such as inner hair cells) and nonsensory cells (such as supporting cells), in an effort to provide new potential therapeutic options for as many people as possible. Our lead program, AK-OTOF, is an AAV gene therapy intended for the treatment of, and potential restoration of hearing in, individuals with sensorineural hearing loss due to mutations in the otoferlin gene (OTOF).
We initiated a Phase 1/2 clinical trial of AK-OTOF for children with OTOF-mediated hearing loss. For information about our clinical trial, please visit otofclinicaltrial.com or contact [email protected].
Minimally invasive transcanal approach
Our Project Pipeline
Current as of: 10/16/2024
AK-OTOF
AK-OTOF is a gene therapy intended for the treatment of, and potential restoration of hearing in, individuals with sensorineural hearing loss due to mutations in the otoferlin gene (OTOF). Normal otoferlin function enables the sensory cells of the ear (hair cells) to release neurotransmitter in response to stimulation by sound to activate auditory neurons.
Without functional otoferlin, auditory signals received by the ear cannot be transmitted to the brain. AK-OTOF uses an adeno-associated viral (AAV) vector to deliver a healthy copy of OTOF to cochlear hair cells, with the goal of restoring long-term physiologic hearing following a single administration to the inner ear. AK-OTOF is intended to treat individuals with sensorineural hearing loss due to mutations in OTOF, who typically have severe to profound bilateral hearing loss from birth, by promoting the expression of normal, functional otoferlin in affected cells of the cochlea. Mutations in OTOF are reported to be a major cause of genetic hearing loss.
We initiated a Phase 1/2 clinical trial of AK-OTOF for individuals with OTOF-mediated hearing loss. For information about our clinical trial, please visit otofclinicaltrial.com or contact [email protected].
For information on expanded access, please visit: Expanded Access and Clinical Trials | Clinical Research | Eli Lilly and Company
AK-antiVEGF
AK-antiVEGF is a gene therapy intended for the treatment of individuals with vestibular schwannoma (VS). Also known as acoustic neuroma, VS is a benign, usually slow-growing tumor arising from Schwann cells surrounding the vestibulocochlear nerve, the nerve that sends information about hearing and balance to the brain. Symptoms of VS may range from hearing loss and imbalance to even more serious neurological problems.
Vascular endothelial growth factor (VEGF) is a protein that promotes the growth of blood vessels and plays a role in VS progression. VEGF inhibitors (anti-VEGF proteins) may slow or reverse the growth of VS and potentially stabilize or improve hearing. AK-antiVEGF uses an adeno-associated viral (AAV) vector to deliver to the cells of the inner ear a DNA sequence that provides instructions to create an anti-VEGF protein; the goal is to achieve local exposure of an anti-VEGF protein in the VS environment that could block VEGF production while limiting systemic effects.
We initiated a Phase 1/2 clinical trial of AK-antiVEGF for individuals with unilateral vestibular schwannoma. For more information about our clinical trial, including the trial design and trial site locations, visit NCT06517888 on ClinicalTrials.gov, or please contact [email protected].